Bioelectrophysics
 Electric field pulses of relatively long duration (microseconds) with relatively long rise and fall times (tens of nanoseconds) and relatively low amplitude (100 kilovolts per meter) produce biological effects at the cellular level primarily as a result of the formation of openings in the external membrane of the cell through the
Electric field pulses of relatively long duration (microseconds) with relatively long rise and fall times (tens of nanoseconds) and relatively low amplitude (100 kilovolts per meter) produce biological effects at the cellular level primarily as a result of the formation of openings in the external membrane of the cell through the 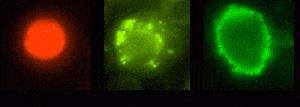 process of electroporation. High-field pulses with widths substantially less than the charging time of the plasma membrane and nanosecond or sub-nanosecond rise times are expressed across intracellular structures and can affect cells in dramatically different ways. Here is a schematic showing differences of electroperturbation and electroporation.
process of electroporation. High-field pulses with widths substantially less than the charging time of the plasma membrane and nanosecond or sub-nanosecond rise times are expressed across intracellular structures and can affect cells in dramatically different ways. Here is a schematic showing differences of electroperturbation and electroporation.
 Membrane Electrophysics — Nanosecond, megavolt-per-meter pulses cause a rearrangement of phospholipids in the cytoplasmic membrane.
Membrane Electrophysics — Nanosecond, megavolt-per-meter pulses cause a rearrangement of phospholipids in the cytoplasmic membrane.
 Intracellular Calcium Distribution — Nanoelectropulses induce release of calcium from intracellular milliseconds of pulse exposure.
Intracellular Calcium Distribution — Nanoelectropulses induce release of calcium from intracellular milliseconds of pulse exposure.
Perturbation of Physiological Homeostasis — Disturbance by ultra-short pulsed electric fields of cytoplasmic compartments, cytoskeletal attachments, nuclear, mitochondrial, and plasma membranes, and the configuration of the endoplasmic reticulum may have specific, dose-dependent effects that are complex and largely uncharacterized functions of pulse width, amplitude, frequency, and pattern.
 Caspase Activation — Unique proteolytic enzymes are exposure. These caspases (cysteine aspartate-specific proteases) are the effectors and executioners of apoptosis (programmed cell death).
Caspase Activation — Unique proteolytic enzymes are exposure. These caspases (cysteine aspartate-specific proteases) are the effectors and executioners of apoptosis (programmed cell death).
Cytological Changes — Nanoelectropulse exposure causes changes in cytoplasmic volume, granulation of the nucleus, and blebbing of the plasma membrane.
Biophotonics
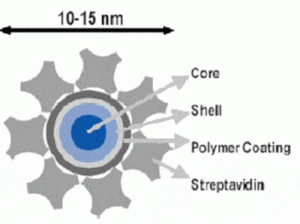
Quantum dot probes are fluorescent colloidal semiconductor nanocrystals. Compared with organic fluorophores, quantum dots have excellent photostability, strong fluorescence, size-tunable emission wavelengths, low toxicity, and long-term chemical stability. These advantages make them extremely proper for long-term cell observations and in vivo tracking. However delivering nanoscale particles into cells and tissues is a challenge for biology research and applications. Two delivery methodes are in progress.
Electropermeabilization — quantum dots were transferred into various cells by the technique of electroporation. Experimental results suggested that the transfer process was partical-size dependent.
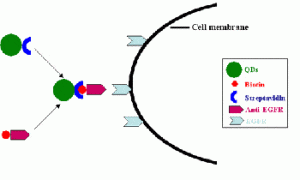
Streptavidin conjugated QDs enter the cells through anti-EGFR mediated endocytosis — Despite aggressive treatment by surgery, radiotherapy, and chemotherapy, the median survival of patients diagnosed with malignant gliomas is less than 12 months. A key variable affecting the survival and quality of life of patients with intracranial gliomas is the extent of tumor resection. In vivo fluorescent spectroscopy and imaging using endogenous and exogenous sources of contrast can provide new approaches for enhanced demarcation of tumor margins and infiltration. The epidermal growth factor receptor (EGFR) is a member of a family of four receptors, which include EGFR (HER1 or ErbB1), ErbB2 (HER2/neu), ErbB3 (HER3), and ErbB4 (HER4). EGFR has been implicated in the development and progression of a number of human solid tumors including brain tumors. Since EGFR is overexpressed in human gliomas, it’s a promising molecular target for quantum dots for tumor demarcation. we investigate the potential use of distinct class of fluorescent molecular probe, quantum dots (QDs), for enhanced optical imaging of brain tumors and intraoperative surgical guidance.
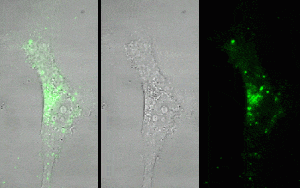
QD-Streptavidin-biotin-anti-EGFR complex can enter human U87 glioma cells and concentrate in the cytoplasm, suggesting that QDs get into cells through anti-EGFR specifically finding to EGFR.
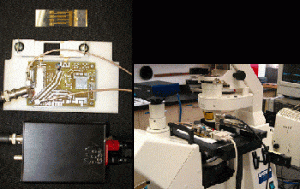
Facility — Real-time Imaging System
A fluorescence microscopy imaging system has been built up for the real-time investigations of electroperturbation and electroporation. An inverted epi-fluorescence microscope coupled with a nanosecond high-voltage pulser, micrometer-scale electrode chambers, and a sensitive CCD camera enable the real-time imaging of intracellular processes during exposures of cells to megavolt per meter electric pulses on a standard microscope glass slide. Custom programs and commercial image acquisition and processing software permit real-time fluorescence imaging and flexible, automated control of pulse parameters. Biological transient responses of Jurkat cells – calcium bursts, phosphatidylserine externalization, and nuclear condensation after nanosecond megavolt-per-meter electric pulses – have been recorded with this system.